Radiation Basics They Should Teach in High School
by Lewis Loflin
Follow @Lewis90068157
Also see What About Humans and Nuclear Radiation?
I know people hate science but here is some anyway. Note that every time I do this I learn something new. Learning is a constant process.
Note this essay does not dismiss the danger of high doses of radiation or careless handling of radioactive material. The issue is unfounded fear and lies from the anti-nuclear activists.
Healthy humans with a good immune system evolved to be immune to low level radiation. This is background material.
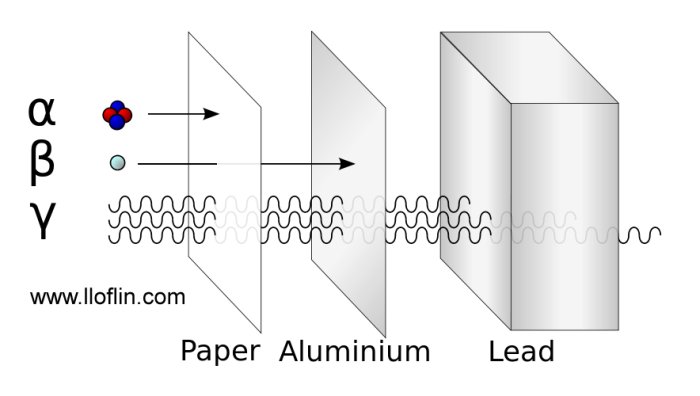
There are three general types of radiation and all originate in the atom nucleus:
Alpha particles are heavy and slow, consisting of two protons and two neutrons. They have a positive charge. They are very ionizing and can cause cell damage.
A few sheets of paper can stop these particles. They are common in smoke detectors. They become helium 4, the helium you breathe in the atmosphere.
Beta particles are high-speed electrons with a negative charge. Very penetrating and can do a lot of damage.
Gamma rays are electromagnetic radiation similar to microwaves but far more penetrating. It takes a few inches of lead or several feet of earth to stop.
Cosmic rays are not electromagnetic radiation but charged particles or ions. They are thrown out by the sun or from across the universe. Ions are atoms stripped of electrons carrying a positive charge. There are also electrons.
These consists of protons, helium 3 nucleus (stellar helium two protons, one neutron) and small numbers electrons, iron ions, etc.
These have different energy levels and can affect biological cells and tissue differently. A healthy immune system is an excellent defense against low-level natural or artificial radiation.
Cosmic rays create a real danger for human space travel. The earth's magnetic field (think northern lights) and the atmosphere shield us from these charged particles.
Note, "The sievert (Sv) is the International System of Units (SI) derived unit of dose equivalent radiation that takes into account the relative biological effectiveness of different forms of ionizing radiation."
Note : 1 REM = 0.01 Sv. = 10 mSv;
0.4 REM = 0.004 Sv or 4 mSv is the background radiation for 1 year in Idaho;
0.3 REM = 0.003 Sv or 3 mSv = 1 Mammogram;
5 REM = 50 mSv maximum occupational dose nuclear workers per year.
Ref. Idaho National Laboratory.

Wild pigs prosper in Fukushima Exclusion zone.
What are "official" safe levels according to www.world-nuclear.org? "In most countries the current maximum permissible dose to radiation workers is 20 mSv per year averaged over five years, with a maximum of 50 mSv in any one year."
According to reports, "Japanese government’s enforced 20 km exclusion zone around the stricken Fukushima Daiichi nuclear power plant is rated at 20 mSv per year."
But this have been proven to be nonsense. Let's us start with feral pigs at Fukushima. Wild pigs are thriving in the exclusion zone and have interbred with domestic pigs. To quote the BBC 30 June, 2021:
"Once people were gone, the boar took over," explains Donovan Anderson, a researcher at Fukushima University in Japan. His genetic study of the wild boar that roam in an area largely abandoned after Japan's 2011 nuclear disaster - has revealed how the animals have thrived.
Using DNA samples, he also discovered that boar have bred with domestic pigs that escaped from farms. This has created wild pig-boar hybrids that now inhabit the zone. "While the radiation hasn't caused a genetic effect, the invasive domestic pig species has," Mr Anderson explained.
One study notes that for Fukushima swine "The median committed effective dose was estimated to be 0.070–0.26 μSv/day for females and 0.062–0.30 μSv/day for males." Assuming a high of 0.3uSv times 365 days equals ~0.11mSv per year, far below the idiotic 20mSv per year some are hysterical about. Yet no apparent genetic or cancer problems.
This comes from a radioisotope known as Cesium-137. This is a chemical cousin to natural potassium which is radioactive. The BBC study and studies at Chernobyl show no apparent genetic or other problems with genetic defects or cancer rates for animals. Swine are a good proxy for humans and this is a real world test not a speculative computer model.
In Ramsar Iran a beautiful biodiversity paradise that has been inhabited by thousands of people for thousands of years, the natural radiation levels are as high as 270mSv per year! Studies show no ill effects on the population. How can this be cry the climate spiritualists?
Research shows most humans and animals in general are immune to low-level radiation natural or man-made. We evolved in a far higher radioactive environment than today. Note the following assuming my math is correct for the last 4.5 billion years:
Half life: K-40 - 1.25 billion yeas; U238 - 4.5 billion years; U235 - 704 million years. 4.5 billion years ago: U238, 4.5/4.5 = 1; 2^1 = 2 times as abundant; K-40, 4.5/1.25 = 3.6; 2^3.6 = 12.13 times as abundant; U235, 4.5/0.704 = 6.39; 2^6.39 = 83.87 times as abundant;
An issue to note is the radioactivity level is directly related to half-life. Half-life is the time a radioisotope decays by 50%. Cesium-137 and Strontium-90 has a half-life of ~30 years while Potassium-40 has a half-life of ~1.25 billion years. Cesium-137 is more radioactive than its natural cousin potassium by far, but decays away at a much faster rate. But radioactive potassium is far more common by orders of magnitude.
In fact common U238 is less radioactive than K40. Thorium is twice as common as uranium and are far less toxic than many common metals such as cadmium and nickel. There is no reason to regulate them other than unfounded hysteria.
| Half Lives | Radioactive | Nonradioactive | Percent Decayed |
| 0 | 1 kg | 0 kg | 0% |
| 1 | 1/2 kg | 1/2 kg | 50% |
| 2 | 1/4 kg | 3/4 kg | 75% |
| 3 | 1/8 kg | 7/8 kg | 87.5% |
To quote one source on Bikini Atoll, "In 1997, researchers found that the dose received from background radiation on the island was between 2.4 mSv/a—the same as natural background radiation." (There is still radioactive isotopes in the soil.)
Let us look at the above decay table and consider the common radioisotopes produced from atomic bombs and nuclear reactors:
Strontium-90 half-life: 28.9 years; Cesium-137 half-life: 30 years, becomes barium-137 half-life 2.6 minutes. Iodine-131 half-life: ~8 days.
Here in 2023 let us consider Bikini Atoll circa 1953, a period of 70 years. Dividing by 30 is 2.33 half-lives. For radioactive strontium and cesium 80% or more has decayed away to harmless isotopes. Iodine-131 all but gone in 5 weeks.
How do "experts" determine safe radiation levels? Or cancer risk levels in general? It is called the Linear No-Threshold or LNT model. This model assumes a lifetime low dose is the same as a massive single, fatal dose.
We know "that high-level radiation could cause immediate physiological effects." But what of low-level doses, intermittent or even constant low-level doses over the years?
To quote, "The decision in the early 1950s was, for administrative simplicity, to assume that the harmful health effects of radiation decreased linearly with radiation dose, all the way down to zero health effect at zero radiation, with no radiation threshold below which we could say there was no significant hazard."
This is nonsense and has been proven so since the 1940s-50s. There are no lingering effects with Japanese nuclear survivors. No lingering problems with most people evacuated from Chernobyl. Ditto plant and animal life.
Studies of residents in regions of high natural radioactivity, including heavy exposure to radon, have shown no abnormal cancer rates, etc., even suggestions to the opposite.
The LNT model is nonsense and needs revision. It is not just radiation but numerous substances they claim "can" cause cancer. No level is ever considered safe.
For example, our blood contains radioactive Potassium 40. To quote, "According to various data, its content in the body of an adult person with body weight 70 kg ranges from 4,000 to 5,000 Bq." (One becquerel is the activity of a quantity of radioactive material in which one nucleus decays per second.)
That is 5,000 nuclear disintegrations per second. Your body emits gamma rays. In other words, gamma rays escaping your body could cause cancer in your baby when holding them.
The radioactive potassium in the banana one has for lunch can cause cancer! Flying across the Atlantic from America to Europe, exposure to cosmic rays can cause cancer! One silly calculation if every person on earth ate a brazil nut, one person would die of cancer from radiation.
Because natural uranium is in seawater, seawater can cause cancer! Others claim chlorinated drinking water "increased risk of colorectal cancers."
Total and utter nonsense. Explain how cancer deaths per 100,000 across the United States have fallen by double-digits.
- Observing Nature in the Real World
- Venus was Never Like Earth Science is Never Settled
- Mastodons Roamed Greenland 2 Million Years Ago
- Present Global Warming Began Circa 1800
- Dissecting Critical Climate Theory
- $1.5 Trillion Climate Change Industrial Complex
- Climate Change Cover for Spiritual & Social Revolution
- Corporate Welfare Grifters Climate Non-Crisis
- When Mythology Replaces Science
- Facts on Hurricanes and Tornadoes Past Present
- Why we should not fear nuclear power.
- Nuclear Graveyards Abound with Life
- What About Humans and Nuclear Radiation?
- Radiation Basics They Should Teach in High School
- How Woke Racism is Killing Education
- What PISA Scores Reveal About Immigration
- Deranged California Teacher Obsesses Over Race
- Lack of Ability is Not Systemic Racism
- Chicago Schools Ruined by Racial Diversity
- High scoring low-income students are white and Asian
- Michigan Muslim-Black Problem in Education
- Electronics, Nuclear Reactors, Applied Science
- Electronics and Technology Built at Home
Bristolblog.com is my other website. I'm a conservationist, I consider ecology a pseudo-religion. Some of the subjects covered:
- Why Learning Programming is Difficult
- Institutionalized Liberal Failure
- What Level of Knowledge for Technology?
- Defining Science and Reason
- Eco-Spiritually is Anti-Science
- Why Do EPA Scientists Oppose Public Disclosure?
- Why Public Disclosure is a Right
- 84% of Americans Fear the Government for Good Reason
- Mechanism Not Spiritualism the Basis of Science
- Eocene Epoch Versus Modern Climate Hysteria
- Off Site:
- Web Master
- Tri-Cities VA-TN
- General Science
- Hobby Electronics
- US Constitution
- Christianity 101
- Religious Themes
Web site Copyright Lewis Loflin, All rights reserved.
If using this material on another site, please provide a link back to my site.

